Why Shouldn't You Mix Vinegar with Bleach?
There’s a wide variety of household products that, for safety’s sake, you should never mix together. Although you may think that combining them “enhances” their cleaning effects, in reality, it can be dangerous. Why shouldn’t you mix vinegar with bleach?
According to Kate Biberdorf, professor of chemistry at the University of Texas at Austin, this is one of the most dangerous pairs in the home. Mixing it releases a toxic gas that’s harmful to your health.
Why do people mix vinegar with bleach?
There are a couple of reasons why people choose to mix vinegar with bleach even though it releases toxic gas. First, vinegar lowers the pH of bleach, which increases its disinfecting action.
However, most people who make these types of mixtures are unaware of the reaction that occurs. And, of course, they’re unaware of the effects that it can cause on health.
So, based only on the desire to make better cleaning products and disinfectants, they make this mixture and use it without precaution.
Don’t miss: Are Cleaning Chemicals Harmful?
What reaction does mixing vinegar with bleach cause?
To understand what happens with this mixture, first of all, we must clarify some concepts. As Dr. Biberdorf points out, atoms make up molecules such as those we find in vinegar and bleach.
In turn, in the center of each atom, there’s a nucleus and, inside it, there are two types of particles: Neutrons and protons. A third type of particle, electrons, orbits the nucleus.
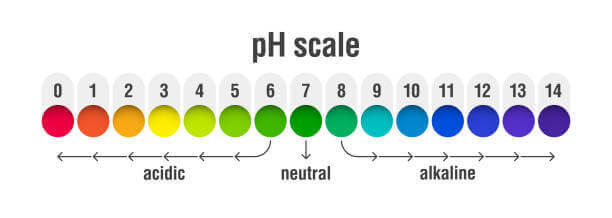
- Acids: Those whose pH is less than 7
- Neutrals: Those whose pH is equal to 7
- Bases: Those whose pH is above 7
When a base and an acid are mixed, a chemical reaction occurs in which the base takes the proton from the acid. This results in a new chemical compound.
So, what happens when you mix vinegar with bleach?
As Biberdorf explains, one of the main components of bleach is sodium hypochlorite, which is a base. Vinegar, on the other hand, is an acid (acetic acid).
Therefore, when a person decides to mix vinegar with bleach, the sodium hypochlorite takes a proton from the vinegar and this reaction produces a hypochlorous acid. However, that’s not the end of the story.
The hypochlorous acid reacts with the rest of the vinegar to form a gas known as chlorine gas, which can be particularly harmful to your health.
Find out more: What to Do if Your Child Drinks Bleach?
The negative effects of chlorine gas
In its pure state, chlorine gas appears to be yellow-green in color. However, the gas that results from mixing vinegar with bleach spreads through the air and is usually invisible.
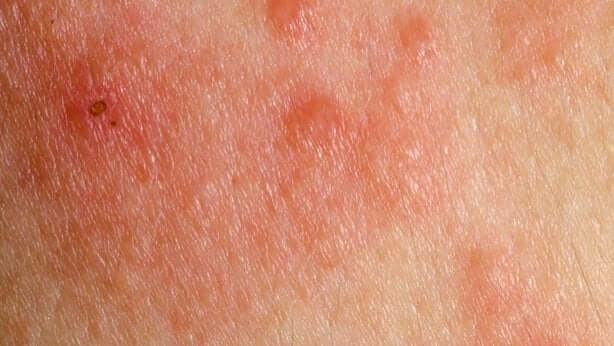
Despite this, you’ll perceive its strong smell and the negative effects it causes in the body. First, it affects the mucous membranes, including the eyes, throat, and lungs.
As a result, symptoms such as watery eyes, chest congestion, breathing difficulties, and a burning sensation, among others, can occur. Also, in case of skin contact, it may cause blisters and irritation.
What can you do to avoid these risks?
Of course, to avoid the above-mentioned risks, you should avoid mixing vinegar and bleach at all costs. Instead, it’s best to use fresh bleach when you want to do any cleaning.
Using bleach and vinegar separately can help disinfect dozens of surfaces. For safety reasons, it’s best to use them individually and only when necessary.
You can also try other natural cleaning alternatives that are safe to use: Lemon juice, baking soda, hydrogen peroxide, and salt are some options.
Had you already heard of the risks of mixing vinegar with bleach? Now that you know that their combination can be very harmful, avoid it completely and share this information with your loved ones.
All cited sources were thoroughly reviewed by our team to ensure their quality, reliability, currency, and validity. The bibliography of this article was considered reliable and of academic or scientific accuracy.
- Morim A, Guldner GT. Chlorine Gas Toxicity. [Updated 2019 Mar 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537213/
- White, C. W., & Martin, J. G. (2010). Chlorine Gas Inhalation: Human Clinical Evidence of Toxicity and Experience in Animal Models. Proceedings of the American Thoracic Society. https://doi.org/10.1513/pats.201001-008SM
- Suryanarayanan, A. (2014). Chlorine. In Encyclopedia of Toxicology: Third Edition. https://doi.org/10.1016/B978-0-12-386454-3.00277-3
- Chlorine Gas Toxicity From Mixture of Bleach With Other Cleaning Products—California. (2011). JAMA: The Journal of the American Medical Association. https://doi.org/10.1001/jama.1991.03470180029010
This text is provided for informational purposes only and does not replace consultation with a professional. If in doubt, consult your specialist.